
Over the last three years, I have accumulated some shares with an average price of $3.9 per share. Here I would like to present my complete esB/R (extended structured Benefit/Risk analysis) investment thesis on ACRX going into Dsuvia PDUFA.
extended Structured Benefit-Risk Analysis
Analysis of condition
The company built both products Dsuvia and Zalviso on the same vision: to replace IV morphine with sublingual sufentanil to treat moderate-to-severe acute pain.
Current Treatment Options
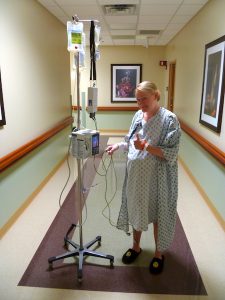
Benefit
The benefit of sublingual sufentanil is from multiple aspects:
Efficacy: fast onset (due to rapid blood:brain equilibration), does not depend on blood flow to muscle, longer duration due to unique PKPD profile of sublingual sufentanil.
Safety: no IV, no chance of infection, no chance of overdosing or underdosing.
Cheaper: no IV setup, save staff time, save ER space.
Convenient: no needle, non-invasive (an irresistible trend in modern medicine), no tethering, which means better mobility for patient's faster recovery.
Just look at these patients, ask yourself, do you prefer being tethered to an IV pole, or roaming around freely?
For more details, please read company website and presentations.
Risk
Opioid crisis
Opioid crisis severely hit pain medication companies. EGLT lost more than 90% its value since 2015. It is developing abuse-deterrent opioid, but could not convince FDA. FDA asked Endo to withdraw Opana ER from market. It seems FDA will approve no new opioid and investors shy away from these companies.
I believe this does not apply to AcelRx and creates huge investment opportunity in the meantime. AcelRx targets the acute pain management in medically supervised setting. It is not chronic home pain management where opioid crisis origins. For more detail, please refer to corporation presentation or this seeking alpha article.
Commercial success
This risk is valid. Given that IONSYS failed to generate any meaningful revenue and now withdrawn voluntarily, AcelRx's new products could for sure face commercial hurdles in the beginning. People in general does not like to change.
I believe, with all the potential benefit of sublingual sufentanil, AcelRx will eventually win physicians and nurses' hearts, and capture a substantial share of IV morphine market, even if it may take some time.
AdCom cancellation
Some people fear that AdCom cancellation is like DVAX's 2nd CRL to Heplisav-B, when DVAX's AdCom was cancelled 1 month after been scheduled. However, it is a different case here. For ACRX, AdCom was not "scheduled then cancelled". It is "implied" at the time of NDA and deemed "no longer plan" after a few months into review.
While most AdCom cancellations were soon followed by CRL, ACRX is still pending review 4 months after AdCom cancellation.
Zalviso device error
Some said market was disappointed by the 2.2% device error rate in the most recent trial. I believe 6 out 320 patients and 7 misplaced pills out of 7,293 dispensed are sufficiently low rates. Most importantly, there was no underdosing (analgesia gap) or overdosing during the trial.
Benefit-Risk Summary Assessment
Overall, the focus should not be error rate or misplaced pills, which are totally manageable.
It should be the pain reduction efficacy, head to head comparison to morphine pump, increased convenience to patients and nurses, its potential market (elderly patients; kinds of operations etc.). Whether these benefits outweigh those manageable risks is the key.
AcelRx's analgesia approach is better in every sense comparing to 100-year-old IV morphine pump. The potential abusing risk is small in medically supervised setting. FDA will be stupid to reject such a superior drug/device for such tiny chance of abuse.
For more details, please read company's Sep 2017 Corporate presentation and Zalviso.eu website (online July 2017; provided by partner Grunenthal).
Understandable first-class business
Long-term enduring economics competitive advantages
In the United States, there is no competition to AcelRx beyond the century old IV morphine pump. In 2015, FDA approved IONSYS. It was a serious competitor to Zalviso.
IONSYS Estimate 1: $500 mil peak sales:
RBC Capital’s Adnan Butt said he expects Ionsys, which is also awaiting a regulatory decision in Europe, to generate worldwide peak sales of about a half a billion in 2022. -- Reuters
Estimate 2: $100-$200 steady earner:
J&J sold it to Incline Therapeutics, which was then acquired by the Medicines Company, and it is forecast to join the same group's Orbactiv and Raplixa as a steady earner in the $100-200m range. -- Epvantage
However, IONSYS failed to generate substantial, if any, revenue for The Medicines Company. As quoted below, IONSYS probably only generated about 2.8mil sales in 2016. No wonder, they voluntarily withdrew IONSYS this year in June 2017.
Other products, including Ionsys, Minocin for Injection, and Orbactiv, along with the divested non-core cardiovascular products, recorded sales of $46.0 million in the full-year 2016 compared to $43.2 million in the full-year 2015. -- The Medicines Company PR.
One can argue, Zalviso or Dsuvia may follow the same commercial fate as IONSYS. I believe sublingual sufentanil will be fundamentally better. This is based on my understanding and opinions from my MD friends.
Partnership
Partnerships from various parties supported AcelRx. They are strong proof of AcelRx's value. These partnerships are:
DOD DARPA Funding: DOD provided at least $22 mil for ARX-04 (Dsuvia) development. They will order 112k doses at the price of $20. You can imagine what if other allies and countries decide to get Dsuvia for their military? Further, will FDA be influenced by DOD demand?
Grunenthal: Zalviso is already approved in Europe in 2015. Grunenthal paid $30 mil upfront, up to $220 mil milestones, and double-digit royalties for only Zalviso in Europe and Australia. In the most recent update (slides #29), the uptake is accelerating.
Ideo, Inc.: legendary design firm IDEO designed Zalviso device. Its design won the prestigeous RedDot design award.
PDL BioPharma: PDLI was willing to pay $65 mil for 75% of royalties and 80% of first four commercial milestones from Grunenthal.
Able and trustworthy management
Pamela Palmer, MD, PhD
Dr. Palmer, a professor at UCSF, founded AcelRx and stayed with the company ever since. She devoted herself to revolutionize acute pain management with sublingual sufentanil. From interview videos, I found her very trustworthy.
Painless Pain Relief: A Non-Invasive Method of Drug Therapy
More videos:
Zalviso.eu website resources: videos.
New CEO: Vincent J. Angotti
His resume emphasize his previous success at XenoPort. He led the re-acquisition of Horizant from GSK and implemented a successful re-branding and re-launch strategy leading to XenoPort's acquisition by Arbor Pharmaceuticals, LLC in 2016.
But I want to point to a detail here. During his 2017Q2 earnings conference call, Angotti answered every analyst's question by calling their name:
"Thanks Dave." "Thank you, Michael." "Thanks Boris." "Thanks Randall."
This is impressive communication skill, suggesting Mr. Angotti pays genuine attention to other people and the conversation. Not your typical biotech CEO with bloated ego.
Other management
Here is another video interviewing AcelRx's CFO Tim Morris and VP of commercial Strategy Ms. Gina Ford.
Small Cap Nation: Company Focus: AcelRx Pharmaceuticals, Inc. (NasdaqGM: ACRX) Feb 2017;
Insider selling:
Major shareholder Perceptive Advisors sold substantial shares over the last 3 years. But insiders are mostly buying shares, except for one recent sell by Larry G. Hamel.
Overall, I found AcelRx has able and trustworthy management.
Bargain sensible price tag
- According to 10-K, ACRX has cash value of $62M. That equals per share value of $1.3.
- IPO price was at $5; recent secondary offering was at $11.65, $3.31.
- Negative responses to phase III data, previous CRL, opioid crisis, FDA drama and management change hit the stock price; no expectation is priced-in until recent pre-PDUFA run .
- Upside estimates: $1B peak sales (700M Dsuvia + 300M Zalviso, about half of company estimates). Market cap $3B (3X P/S reflecting modest valuation with no follow-on pipeline). ACRX now has
46mil60.6mil shares. We estimate after full dilution and secondary offering, it will reach up to60mil75mil shares. We predict ACRX will be bought out for about$50$40/share around 2019-2020, when the company receives approval on both products and proves its popularity among providers and patients.
As of 09.2017, at $3~5/share, it is less than the price of IPO and later offerings. AGRX sees 60-80% downside risk to liquidation value while offering potential 10X upside upon approval.
Conclusion
Better proven drug, better device, better route. AcelRx's sublingual sufentanil analgesia platform has better efficacy (fast onset, does not depend on blood flow to muscle), safer (no IV complications, no needle, no overdosing, no dosing gap), cheaper (IV cost, legal cost, staff time coast, ER bed cost) and more convenient (no needle, no IV pole, better mobility). I believe FDA will approve these two paradigm shifting products.
With visionary co-founder Dr. Pamela Palmer and experienced CEO Angotti, as well as a stock price repressed by negative sentiments surrounding previous CRL, opioid crisis and doubts, ACRX presents substantial investment return in the next 2-3 years.
