
Disclaimer: I own shares of DVAX.
Update 2018.11.30: I was wrong with the speculation. AZD1419 Ph2a trial did not meet primary endpoint. FierceBiotech news.
Original Post:
Today I would like to speculate on the on-going Phase IIa trial of AZD1419. It will likely be a success.
I have previously written a blog post about the unique MOA and delivery route of AZD1419. Unlike existing steroid, β adrenoceptor agonists or immunosuppressant drugs, AZD1419 is the first true immunomodulatory drug candidate for asthma.
Lots of immunosuppressant drugs that block the asthma cascade are often called immunomodulatory. But they simply suppress the signaling or cytokine cascade after symptoms start. They don't really modulate or rebalance the lung immune system to prevent asthma from happening again. This is what AZD1419 (via CpG-TLR9 pathway) is designed to do: AZD1419 is designed to be a cure, not a drug that patients have to take for entire life. Read more at my post and AstraZeneca webpage.
This human trial has a withdrawal design. Patients were withdrawn from LABA after 7 weeks, then ICS to be on AZD1419 monotherapy, then completely drug free for follow-up till up to 52 weeks.
fairvalueforyou made a very interesting logic speculation on IV:
"Why I am expecting some positive news from AZD1419 phase IIa trial result" by fairvalueforyou @IV
Here is the link to his post @IV. His argument is quoted below:
Please note, AZD1419 phase IIA enrollment was completed on 9/21/2017, and the trial is still active today and will be completed by 9/24/2018. Therefore, some participants had/have not met the primary endpoint(loss of asthma control) and continued/continue their once weekly monotherapy AZD1419/placebo inhalation after initial 13 dose treatment. In other words, AZD1419 phase IIA trial would have been finished soon after initial 13 dose treatment period if neither AZD1419 nor placebo worked. Could placebo somehow have worked for some patients up to 52 weeks without asthma medications? Zero chance! And it has to be AZD1419 using its magic to keep some patients to stay in this trial up to 52 weeks without asthma medication. (I want to know how many patients being able to take AZD1419 without asthma medication up to 52 weeks now!)
I could not see any possibility that AstraZeneca keeps AZD1419 phase IIA trial active for a year after completing its enrollment without participants failing to meet primary endpoint up to 52 weeks
We will know AZD1419 result soon in later September or earlier October, and good luck to all
Note: he misunderstood the trial design detail. There is no drug at all after 13 weeks. Again, AZD1419 is designed to be a cure.
My further speculation
Following his clue, I replied with further speculation:
In July they moved completion date from 28 to 24th, it is because someone who was supposed to finish 52 week by 28th met the endpoint of loss of control and left the trial. This patient was the very last randomized recruitment in late September 2017. He/she could be a placebo or AZD1419. He might have a couple of months (up to 6 months) asthma control.
More important, this also means someone who supposed to finish on 24th are still going strong without loss of control, at least in July. That is half a year after last dose of AZD1419.
The closer we get to Sep 24th, the longer lasting AZD1419 works, and the larger potion of patients don't even meet the endpoints at all.
Let's put away speculations for now and look for some evidences:
What is "moderate to severe" asthma?
According to references (1, 2), Asthma is classified as moderate (severe) persistent if symptoms occur daily (and more often). They require daily medication ICS+LABA according to this NIH guideline (steps 3 and above):

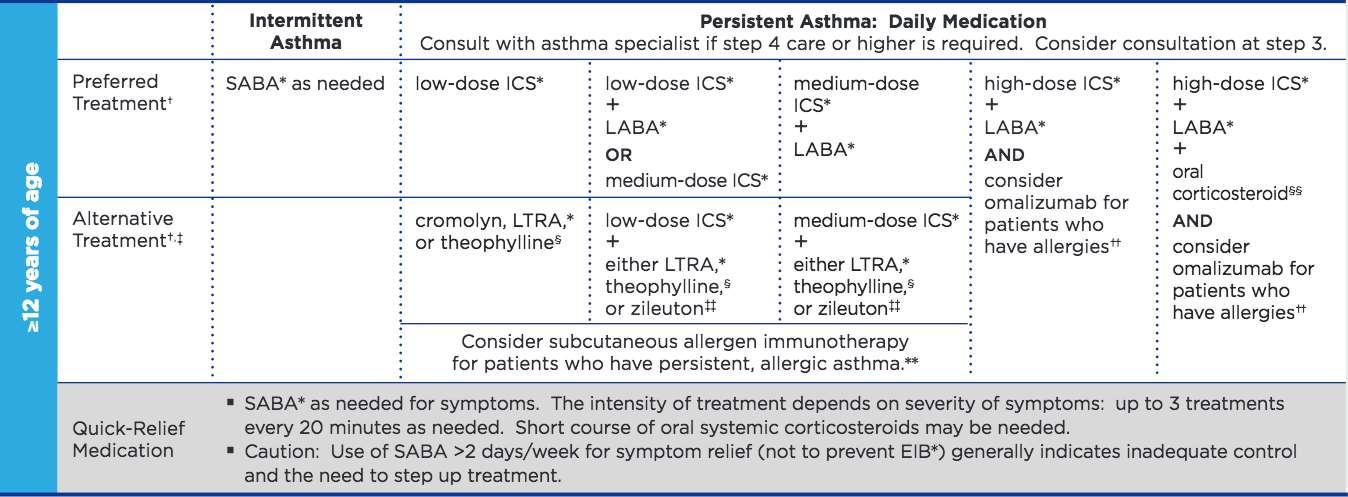
What does scientific literature say?
Then I looked into biomedical literature to find out how long does it take for "moderate to severe" asthma patients to lose control?
I found 6 published human clinical trials. You can review the summary of data below:
| Trial | Year | Journal | Patients | Max follow up | Percent LOC | Time to loss of control |
|---|---|---|---|---|---|---|
| Gibson et al. | 1992 | Clin. Exp. Allergy | mild | 27 days | 100% | mean 16d (to exacerbation) |
| Jones et al. | 2000 | Am J Respir Crit Care Med | mild to moderate (ICS only) | 6 week | 78% | median 17 d (95% CI: 14, 28) |
| Jatakanon et al. | 2001 | Am J Respir Crit Care Med | mild to moderate (ICS only) | 8 week | 47% | median 8wk |
| Belda et al. | 2006 | Can Respir J. | mild to moderate (ICS only, 2 w.LABA) | 21-69 days | 53% | mean 33d |
| Maneechotesuwan et al. | 2007 | Chest | moderate (ICS only) | 10 week | 67% | median 6wk (to exacerbation) |
| Sneeboer et al. | 2015 | Clin. Exp. Allergy | moderate to moderately severe (17/22 with LABA) | 8 week | 96% | median 22 d |
| AZD1419 | 2018 | moderate to severe (100% w. LABA) | 39 week | ?? | ?? |
The last one, Sneeboer et al. 2015, paper is closest to AZD1419 trial in patients cohort, and still not every one uses LABA. LABA usage is required for AZD1419 trial. Nonetheless, in Sneeboer trial, 96% (22/23) of patients lost asthma control in 8 weeks with 22 days median time to loss of control.
In Sneeboer trial, 96% (22/23) of patients lost asthma control in 8 weeks with 22 days median time to loss of control.
P.S., as pointed by fairvalueforyou in a reply, CYT003, the failed intravenous (thus systemic) CpG treatment for asthma failed 5 months after enrollment complete (7-week treatment +12-week follow up). The plan was 9-month follow-up. The trial had an add-on design, not withdrawal. Patients had ICS with or without LABA. [Links: Trial, PR, and Paper]
What can we infer for AZD1419 trial?
I will conclude that with a cohort of all LABA patients, the placebo arm will all lose asthma control within 8 weeks, which is by February 28, 2018.
According to ClinicalTrials.gov records, AstraZeneca submitted two trial updates in 2018: 3/28/2018 and 7/5/2018. We are now at 8/15/2018, the trial is still ongoing.
If AZD1419 has no effect, the trial should have ended by March update. The trial is still ongoing and worth the money spent to keep it going by at least 7/5/2018. It suggests that a significant portion of patients are still going strong without loss of asthma control.
And they cannot be placebo patients.
Move from 9/28 to 9/24
Another interesting thing in this speculation is the 4 day move of primary completion dates at 7/5/2018 ClinicalTrials.gov update.
This trial enrolled about 170 patients (81 in active arm). Consider ramping up, I estimate in the end of enrollment around September 2017, they could have enrolled 14-28 patients per month, about 1-2 days per patient.
This is speculation in extreme here:
Moving from 9/28 to 9/24 (4 days) suggests 1-4 (out of last 2-5) patients could have lost control at 7/5/2018 update. It also suggests 20-50% AZD1419 patients are still going strong at 7/5/2018, which is about 26 weeks therapy free.
Of course, the 4-day change may be just because of some administrative reasons.
Nonetheless, the trial is still going. Some patients have not loss asthma control. Let's wait and see. And the longer we wait, the better the results.
Conclusion
In short, fairvalueforyou and I expect positive results from AZD1419. Specifically, I expect 20-50% patients stay controlled and therapy free beyond 26 weeks after withdrawal in the active arm, while all placebo lose control within 8 weeks after withdrawal.
There is potentially a group of patients stay controlled the entire 39 weeks of follow-up, which suggests a remission for moderate to severe asthma.
Let us wish Nobel prize-winning CpG-TLR9 pathway bring a better therapy for Asthma patients! Happy speculating!
